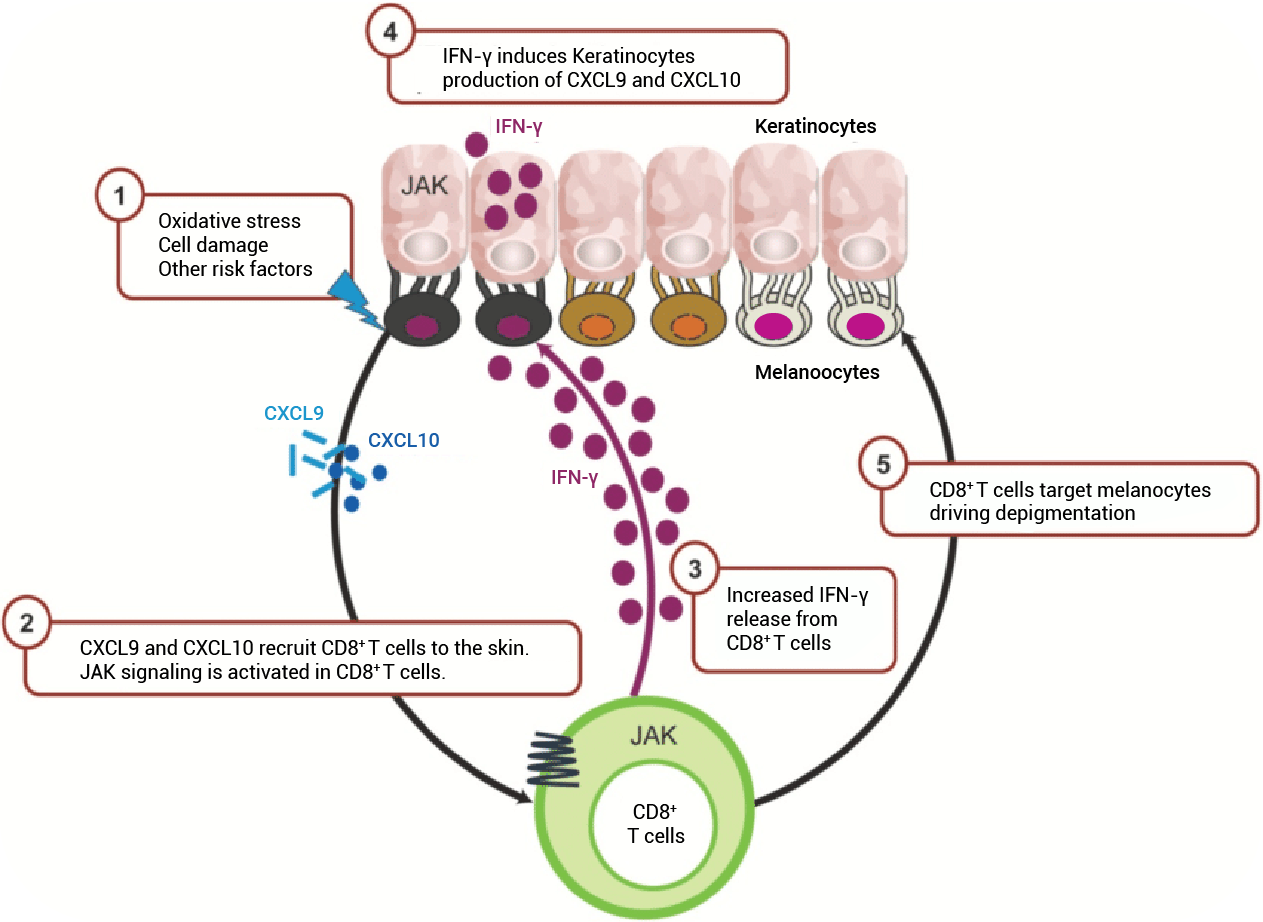
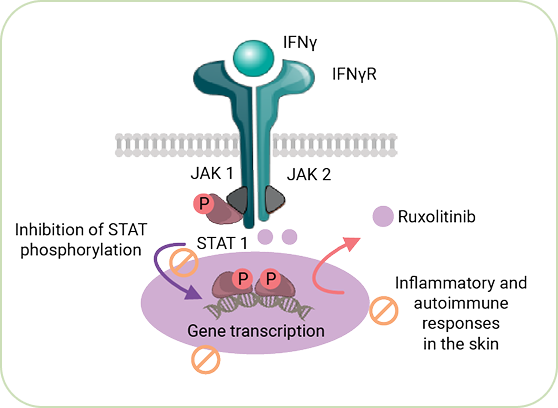
Ruxolitinib Efficacy
Data from Pivotal Trials


Two Phase 3, double-blind, vehicle-controlled trials Topical Ruxolitinib Evaluation in Vitiligo Study 1 [TRuE-V1] and [TRuE-V2] examined the efficacy of ruxolitinib cream for repigmentation of skin lesions in adolescents and adults with nonsegmental vitiligo.
This was a two phase 3, double-blind, vehicle-controlled trials in North America and Europe that involved patients 12 years of age or older who had nonsegmental vitiligo with depigmentation covering 10% or less of total body-surface area.² Patients were randomly assigned in a 2:1 ratio to apply 1.5% ruxolitinib cream or vehicle control twice daily for 24 weeks to all vitiligo areas on the face and body, after which all patients could apply 1.5% ruxolitinib cream through week 52.²
TRuE-V1 and TRuE-V2 Study Design ⁹
*1 randomized patient who did not apply ≥1 dose of Ruxolitinib cream was excluded from safety analyses.
13 patients from 1 study site were excluded from efficacy analyses for compliance issues.
Primary Endpoint
- % of patients achieving an F-VASI75 response at week 24.
Key Secondary Endpoints (all at week 24) ²
-
% of patients achieving F-VASI50 and F-VASI90 responses.
% of patients achieving a T-VASI50 response.
% of patients achieving a VNS response of 4 ('a lot less noticeable') or 5 ('no longer noticeable').
% change from baseline in affected F-BSA.
Other Secondary Endpoints ²
-
Safety and tolerability.
% change from baseline in F-VASI, T-VASI, affected F-BSA and T-BSA during the treatment period.
% of patients having F-VASI improvements or T-VASI improvements.
The face includes the area on the forehead to the original hairline, on the cheek to the jawline vertically and laterally from the corner of the mouth to the tragus. It includes the surface area of the nose but not that of the lips, scalp, eyelids, ears, or neck.² VNS was assessed for facial lesions only.²
Patient Demographics and Clinical Characteristics ²
Baseline demographics and clinical characteristics were similar for TRuE-V1 and TRuE-V2.
References
- 2. Rosmarin D, Passeron T, Pandya AG, Grimes P, Harris JE, Desai SR, Lebwohl M, Ruer-Mulard M, Seneschal J, Wolkerstorfer A, Kornacki D. Two phase 3, randomized, controlled trials of Ruxolitinib cream for vitiligo. New England Journal of Medicine. 2022 Oct 20;387(16):1445-55.
- 9. Rosmarin D, Passeron T, Pandya AG, Grimes P, Harris JE, Desai SR, Lebwohl M, Ruer-Mulard M, Seneschal J, Wolkerstorfer A, Kornacki D. Two phase 3, randomized, controlled trials of ruxolitinib cream for vitiligo. New England Journal of Medicine. 2022 Oct 20;387(16):1445-55. Supplementary material.