Safety Profile
Look into the safety data


Ruxolitinib cream was well-tolerated.
Treatment-related TEAEs among patients who applied Ruxolitinib cream at any time were all mild or moderate (none serious)
Adverse Reactions Associated with Ruxolitinib at 52 Weeks⁹
Adverse Reactions Occurring in Patients Treated with Ruxolitinib through Week 52 In TRuE-V1 and TRuE-V2
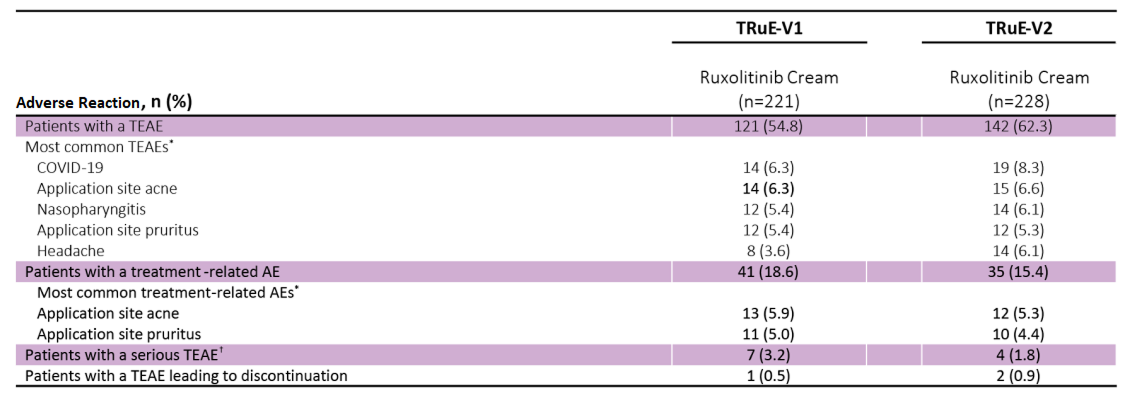
Occurring in ≥2% of patients in any treatment group.
No serious TEAEs were considered by the investigators to be related to treatment.
Reference:
- 9. Rosmarin D, Passeron T, Pandya AG, Grimes P, Harris JE, Desai SR, Lebwohl M, Ruer-Mulard M, Seneschal J, Wolkerstorfer A, Kornacki D. Two phase 3, randomized, controlled trials of ruxolitinib cream for vitiligo. New England Journal of Medicine. 2022 Oct 20;387(16):1445-55. Supplementary material.