Efficacy PROFILE
Explore Lumirix Efficacy


Half the patients who applied Ruxolitinib reached F-VASI75 at week 52.
Efficacy of Ruxolitinib Application on the Primary Endpoint F-VASI75 Response.
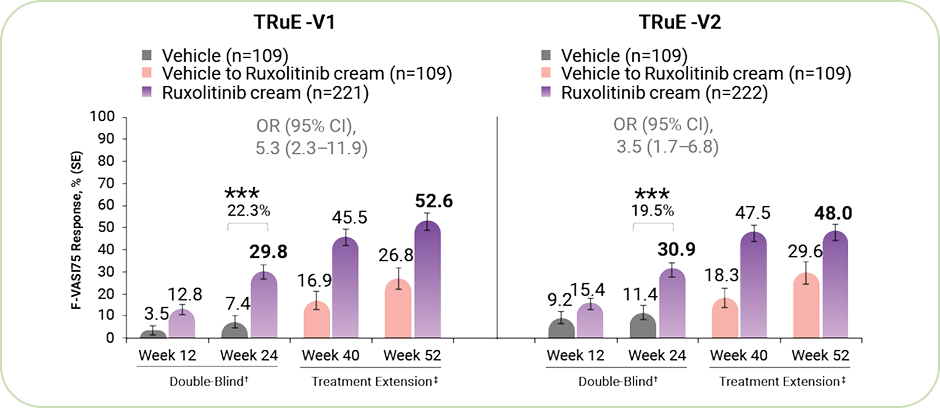
ᵃ Percentage calculated from pooled figures of the identical TRuE-V1 and TRuE-V2 study, rounded off to the nearest whole number.
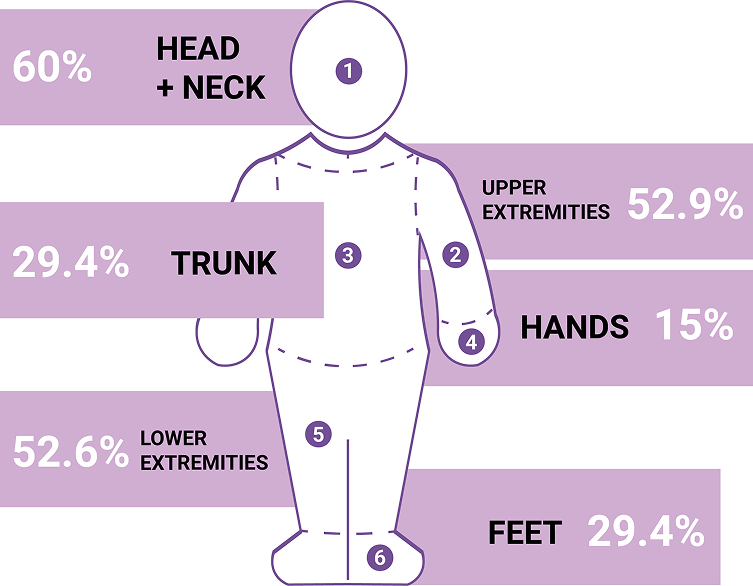
Adapted from ref. 11
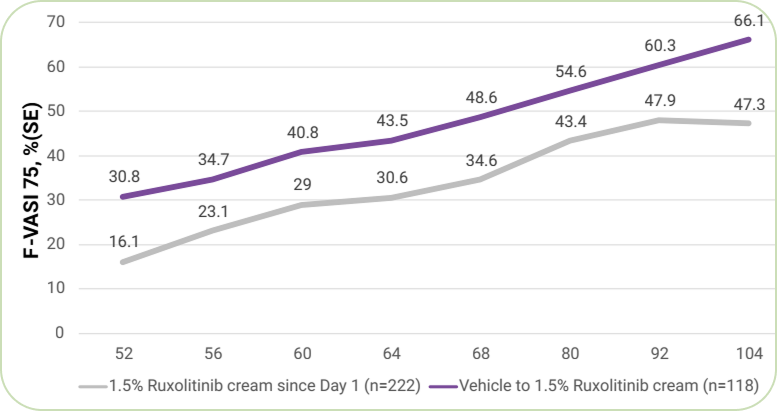
63.8%
of patients who applied Ruxolitinib cream since day 1 achieved F-VASI75 at week 104 (LTE end-of-treatment).
Reference:
- 2. Rosmarin D, Passeron T, Pandya AG, Grimes P, Harris JE, Desai SR, Lebwohl M, Ruer-Mulard M, Seneschal J, Wolkerstorfer A, Kornacki D. Two phase 3, randomized, controlled trials of Ruxolitinib cream for vitiligo. New England Journal of Medicine. 2022;387(16):1445-55
- 9. Rosmarin D, Passeron T, Pandya AG, Grimes P, Harris JE, Desai SR, Lebwohl M, Ruer-Mulard M, Seneschal J, Wolkerstorfer A, Kornacki D. Two phase 3, randomized, controlled trials of ruxolitinib cream for vitiligo. New England Journal of Medicine. 2022 Oct 20;387(16):1445-55. Supplementary available at: https://www.nejm.org/doi/suppl/10.1056/NEJMoa2118828/suppl_file/nejmoa2118828_appendix.pdf. Last accessed: 4.12.2024.
- 11. Hamzavi I, et al. 'Efficacy of Ruxolitinib Cream in Vitiligo by Patient Characteristics and Affected Body Areas: Descriptive Subgroup Analyses from a Phase 2, Randomized, Double-Blind Trial'. Journal of the American Academy of Dermatology, 2022;86:1398–401